.png)
If your company already has a quality management system in place, you’ve cleared one of the biggest hurdles on the path to a compliant medical device. Most regulators, however, expect that system to meet ISO 13485 before your product can reach the market. Here’s how to bring your QMS in line with the standard without losing sight of how your team actually works.
Start by getting a feel for the core building blocks of ISO 13485. A quick overview sits in the article ISO 13485 – Introduction, General Understanding and the Role of Training in the Medical Technology Sector
What matters most is to understand why ISO 13485 exists in the first place: it protects patients by making sure medical devices come from stable, well-controlled processes. Just as important is the role of training and daily quality awareness — the two things that stop a QMS from being a stack of documents and turn it into something people actually use.
A medical device only reaches high quality when quality thinking sits deep in the culture. Every single process leaves a mark on the final product. If one link weakens, the overall quality slips with it. That’s why everyone in the company needs to see quality as a guiding principle, not a box to tick. When the mindset shifts, teams stop treating procedures as chores and start noticing when something feels off: a delay, a mismatch, a risk creeping up. That awareness is priceless.
Leadership has to set the tone. Management needs to speak openly about quality, explain why specific goals matter, and model the behaviour they expect. A CEO who holds short quality huddles — where wins and struggles come on the table without judgement — makes transparency normal and shows commitment from the top down. Open communication, early problem-solving and a supportive response to findings strengthen that culture further. Ongoing training tied to each role helps bring the values of quality management into everyday decisions.
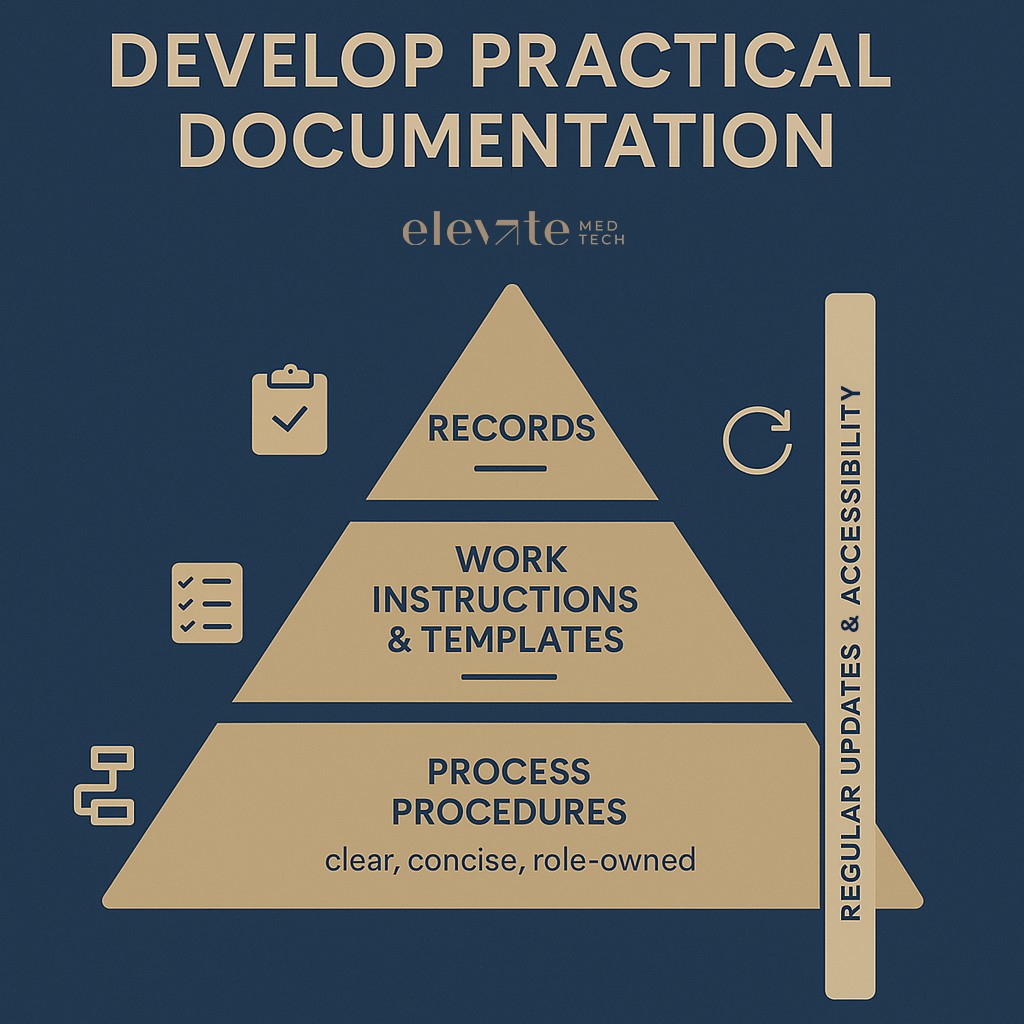
Documentation needs to meet ISO 13485 requirements, but it should also work on a normal Tuesday morning. The process owner should lead each process. They’re the people who know their field best and are responsible for describing and maintaining how things run.
Keep procedures clear, short, and scaled to your company’s size and resources. To ensure clarity and efficiency, structure each work instruction or process as a sequence of well-defined process steps. Each step should specify the required input, the activity to be performed (including the responsible person), and the expected output. Furthermore, assign a dedicated process owner to every process to maintain responsibility and oversight. Focus on records that show compliance and genuinely help the team. Make sure documents are easy to find and don’t gather dust. And resist the trap of writing a small novel for every process. Tailor your QMS to the scale and complexity of your startup. Avoid paperwork for paperwork’s sake and protect the energy you need for the things that truly drive patient safety.
Quality doesn’t live in a single department. Assign responsibilities for quality tasks across the organisation and make sure accountability sits where the work happens. Sometimes this means people step into additional roles that support quality and regulatory needs. A software developer might also serve as software architect in the development plan, even when the official job title doesn’t mention it. These overlaps are normal in medtech and help a young company stay nimble without losing control.
Products and processes reach steady quality only when both the QMS and the daily routines follow clearly defined paths. That works best when every critical step runs in a consistent sequence. Procedural controls form the backbone of that approach. They outline how a process starts, who’s involved, what happens next, and how results are captured.
These controls shape all core areas: design, production, supplier management and the handling of deviations or repeated issues (CAPA: Corrective & Preventative Actions). Each of these tasks needs a procedure that explains how the team works and how it keeps risks in sight. Well-written procedures bring order to complex activities. They stop people from reinventing the wheel or skipping steps under pressure. Documented processes create a shared standard that reduces variation and makes outcomes more predictable, a key expectation under ISO 13485.
Most major regulatory frameworks require a proper QMS for medical devices. ISO 13485 helps you meet those expectations, but the actual legal requirements — such as those from the FDA or EU MDR — remain the decisive factor for market access. With a few smart tweaks, however, a single ISO 13485-aligned QMS can cover several regions at once. Stay up to date with regulatory changes and weave them back into your system as they arise.
Internal audits reveal what already works, what doesn’t, and whether your system truly meets ISO 13485. An internal audit is a structured, objective and well-documented check of how well your QMS aligns with standards and regulations. It uncovers gaps, prepares the team for external audits and is worth its weight in gold.
Run internal audits regularly. Treat findings as moments to learn, not as failures. Turn audits into part of the company rhythm, something that keeps the system sharp. For more on the role of internal auditors, the article The Internal Auditor: A Key Role in Quality Management by Elevate MedTech offers a solid deep dive.
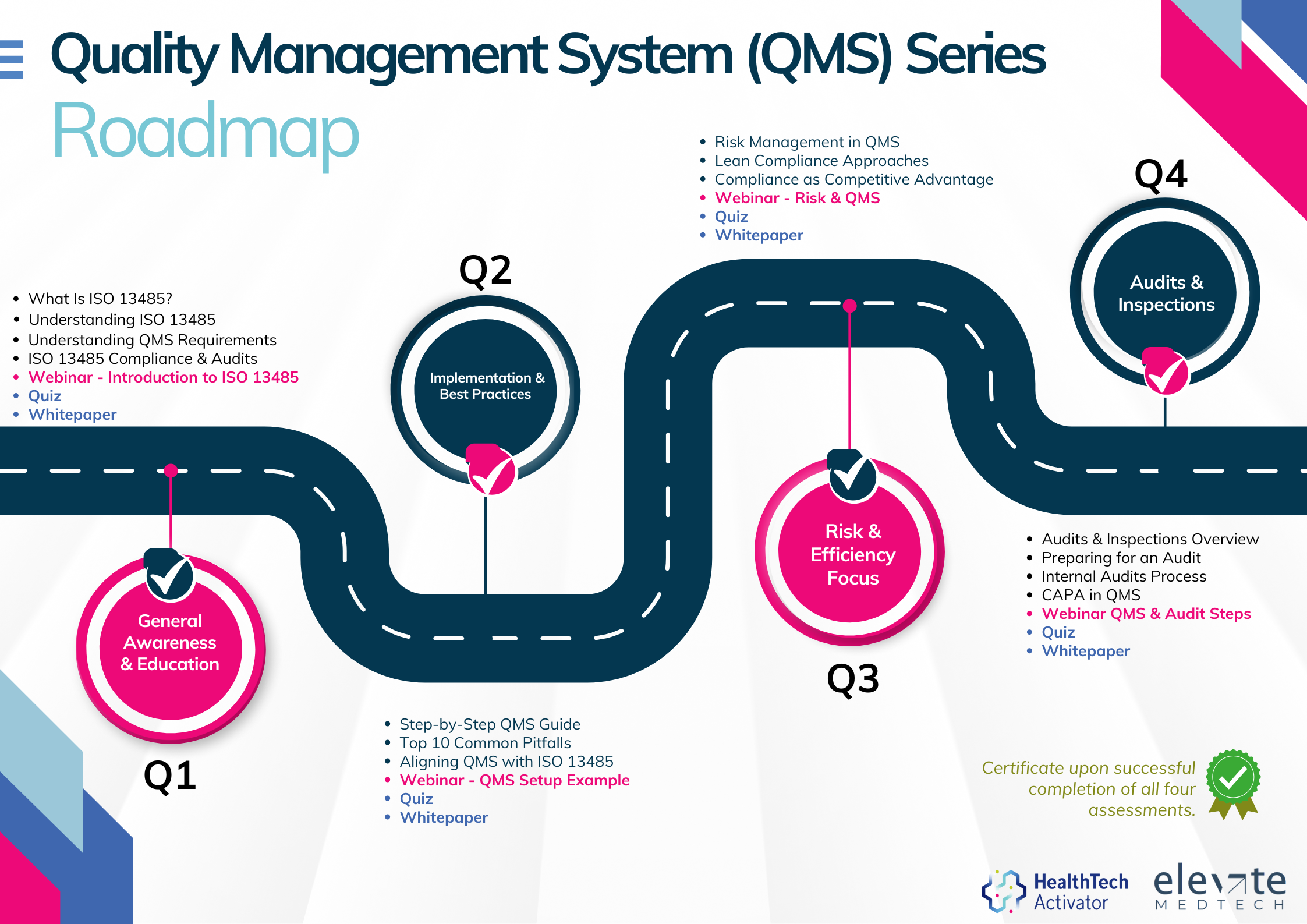
Meeting the requirements of ISO 13485 is one thing, putting them into practice is another. That’s why this article is part of a broader series developed for New Zealand’s HealthTech sector, aimed at helping teams turn regulatory expectations into working systems.
Over 12 months, this series explores ISO 13485 in four parts: from first steps and system setup to risk management and audit readiness. Each quarter combines practical content with interactive workshops to support implementation in real-world settings.