
ISO 13485 – Introduction, General Understanding and the Role of Training in the Medical Technology Sector
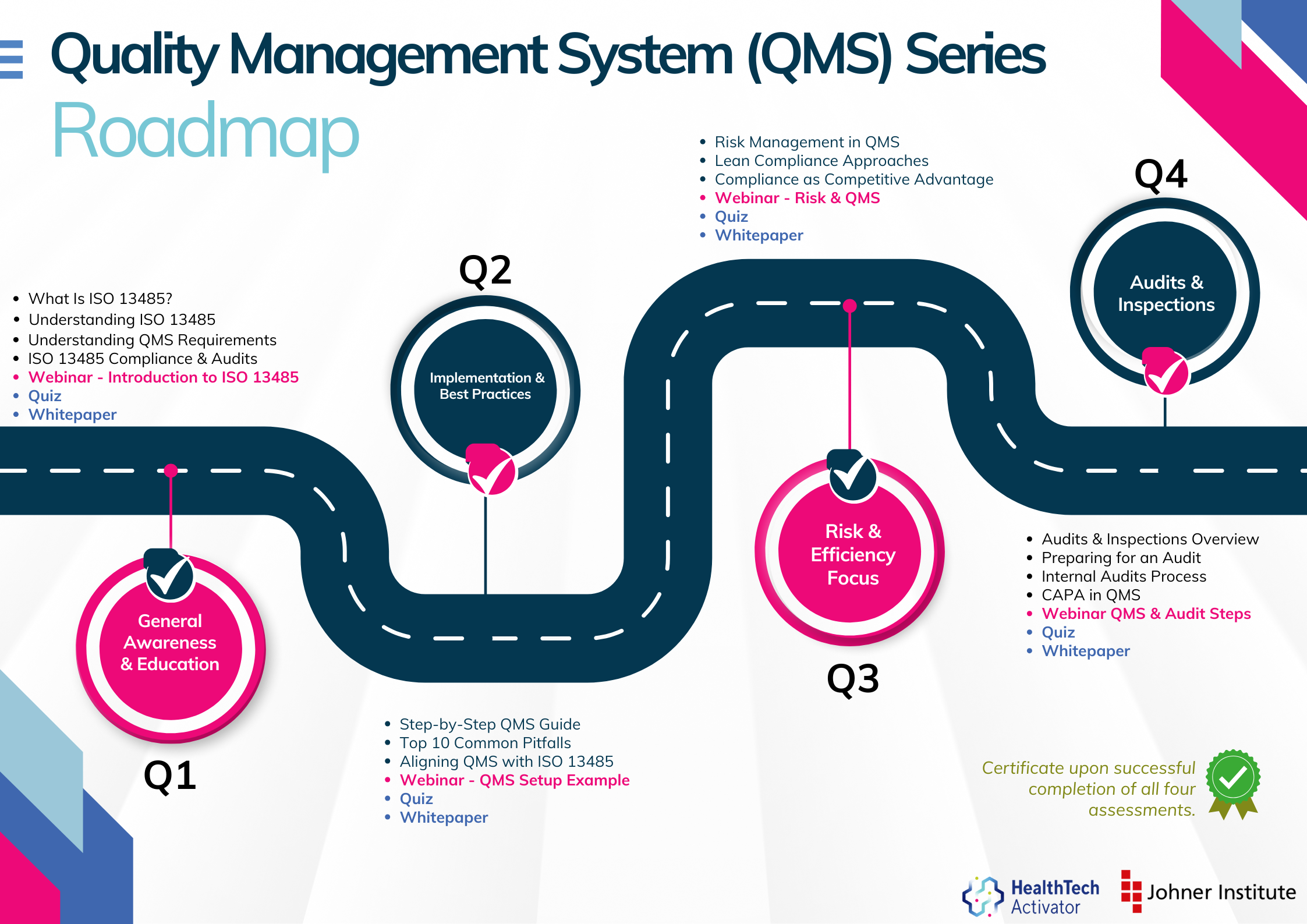
QMS Series - Article 1 - ISO 13485. This article outlines the key elements of ISO 13485, explains its role in regulatory compliance, and explores how staff training and general quality awareness are essential to ensuring the system works in practice.

ISO 13485 – Introduction, General Understanding and the Role of Training in the Medical Technology Sector
QMS Series - Article 1 - ISO 13485. This article outlines the key elements of ISO 13485, explains its role in regulatory compliance, and explores how staff training and general quality awareness are essential to ensuring the system works in practice.

Understanding ISO 13485: A Practical Guide for Small Medical Device Companies
QMS Series - Article 2 - ISO 13485. This article explains how to think about ISO 13485 practically, particularly from the perspective of smaller companies and startups with limited resources. The focus is on developing a practical and pragmatic approach to quality that aligns with business realities.

Understanding ISO 13485: A Practical Guide for Small Medical Device Companies
QMS Series - Article 2 - ISO 13485. This article explains how to think about ISO 13485 practically, particularly from the perspective of smaller companies and startups with limited resources. The focus is on developing a practical and pragmatic approach to quality that aligns with business realities.

Understanding QMS Requirements Under ISO 13485:2016
QMS Series - Article 3 - ISO 13485. This article explains the core requirements of ISO 13485, including documentation, responsibilities, procedural controls, and how the standard connects to wider regulatory obligations.

Understanding QMS Requirements Under ISO 13485:2016
QMS Series - Article 3 - ISO 13485. This article explains the core requirements of ISO 13485, including documentation, responsibilities, procedural controls, and how the standard connects to wider regulatory obligations.

ISO 13485 Compliance: What You Need to Know Before Your Next Audit
QMS Series Article 4 - ISO 13485. This article outlines how to prepare your quality management system for the next assessment — and how to approach the process with confidence rather than stress.

ISO 13485 Compliance: What You Need to Know Before Your Next Audit
QMS Series Article 4 - ISO 13485. This article outlines how to prepare your quality management system for the next assessment — and how to approach the process with confidence rather than stress.


.png)




.png)