A functioning Quality Management System (QMS) compliant with ISO 13485 is the foundation for gaining market approval of any medical device worldwide. Yet many companies waste time and resources by taking the wrong approach to implementation. To help you avoid the frustration, here are the ten most common pitfalls when building an ISO 13485-compliant QMS – and how to steer clear of them.

The most frequent mistake when developing a QMS is to treat it as a checklist exercise for isolated departments rather than an integral part of the company culture. Ticking off individual ISO 13485 requirements might seem sufficient at first, but if quality is not embedded in every workflow and at every level, non-compliance is only a matter of time, and correcting it will be costly.
When every employee sees quality as part of their daily work, the entire QMS runs more smoothly, requires fewer corrections, and ultimately results in safer, higher-quality products.
The key to success: active involvement and visible commitment from management. Leaders must set the example.
What usually happens when management designs processes without involving the people who actually carry them out? Exactly — it backfires. No one understands what works in practice better than the process owners.
That’s why it’s essential to involve the team when creating or revising the QMS. Otherwise, companies end up with systems that might look fine on paper but fail in everyday use. Missing alignment between SOPs and real workflows leads to poor employee adoption and weak QMS performance.
This ties directly to the previous point. Companies that don’t think about practicality while writing procedures will soon notice that no one follows them. It’s great to aim high, but if a process becomes so complicated that staff ignore it, the result is worse than a simpler, more realistic approach.
- Involve teams when designing procedures
- Build on what already works instead of reinventing everything
- Run small test cycles to see whether the process holds up in reality.
Simple, well-structured processes will always outperform complex ones that sit unused.
A structured approach is essential when drafting SOPs. Without it, documents often become inconsistent, redundant, or overly detailed, making them hard to implement and maintain. Each procedure should clearly define its purpose, inputs, outputs, and interactions with other processes.
A structured process approach keeps documentation clean, easy to follow, and adaptable. It also makes training easier, and auditors much happier.
Every process interacts with others. If these links aren’t clearly identified and documented, the entire system loses coherence. For example, design control influences purchasing, which in turn affects production and post-market surveillance. If those connections aren’t mapped, information slips through the cracks, and quality suffers.
ISO 13485 requires a process-based approach for exactly that reason. Documenting how processes connect ensures transparency, better decision-making, and a smoother flow of information across the company.
In emergencies, people are advised not to shout for help to everyone, but to address someone directly, because when a request goes to all, no one reacts. It’s exactly the same with QMS tasks.
Without clear ownership, work simply doesn’t get done. A QMS needs precise role definitions and personal accountability for each task. Every activity should have a name beside it, someone who knows what they’re responsible for and can act on it.
This is particularly important beyond the Quality and Regulatory (Q&R) function. If responsibilities stay only within one team, cross-functional cooperation breaks down and the system weakens. Clear roles make accountability visible and turn quality into a shared responsibility across the company.
ISO 13485 rightly requires staff training. But how that training happens matters even more. It’s not enough for employees to know the general outline of a process, they need to understand it. They should know how the different parts of the QMS connect and why each step matters for safety and compliance. Just as important is to weave the QMS culture into every session, so that quality becomes part of daily work rather than something separate from it.
Anyone who thinks they can build a QMS once and then forget about it will eventually get an unpleasant surprise. A QMS is a living system that must keep evolving with changes in the company, in the products (including customer feedback!) and in the regulatory landscape. Regular reviews are essential. They answer key questions:
- What works well?
- What doesn’t?
- What has changed since the last review?
Fixed review intervals — and sticking to them — ensure that the QMS stays relevant and effective. Without them, the system quickly drifts away from reality.
ISO 13485 places strong emphasis on risk management. Ignoring risk-based thinking means missing vital opportunities to focus resources where they matter most: on patient safety and compliance. When designing QMS processes, risk assessment should guide decisions at every stage. This approach not only minimises potential non-conformities but also strengthens product quality and reliability.
Document control may sound like a dull administrative task but underestimate it and chaos quickly follows. Without proper version management, outdated or conflicting SOPs keep circulating, leading to errors and non-compliance.
A strong document control system ensures everyone works with the latest approved version, changes remain traceable, and obsolete files are properly withdrawn. It’s one of the simplest yet most powerful safeguards for maintaining QMS integrity.
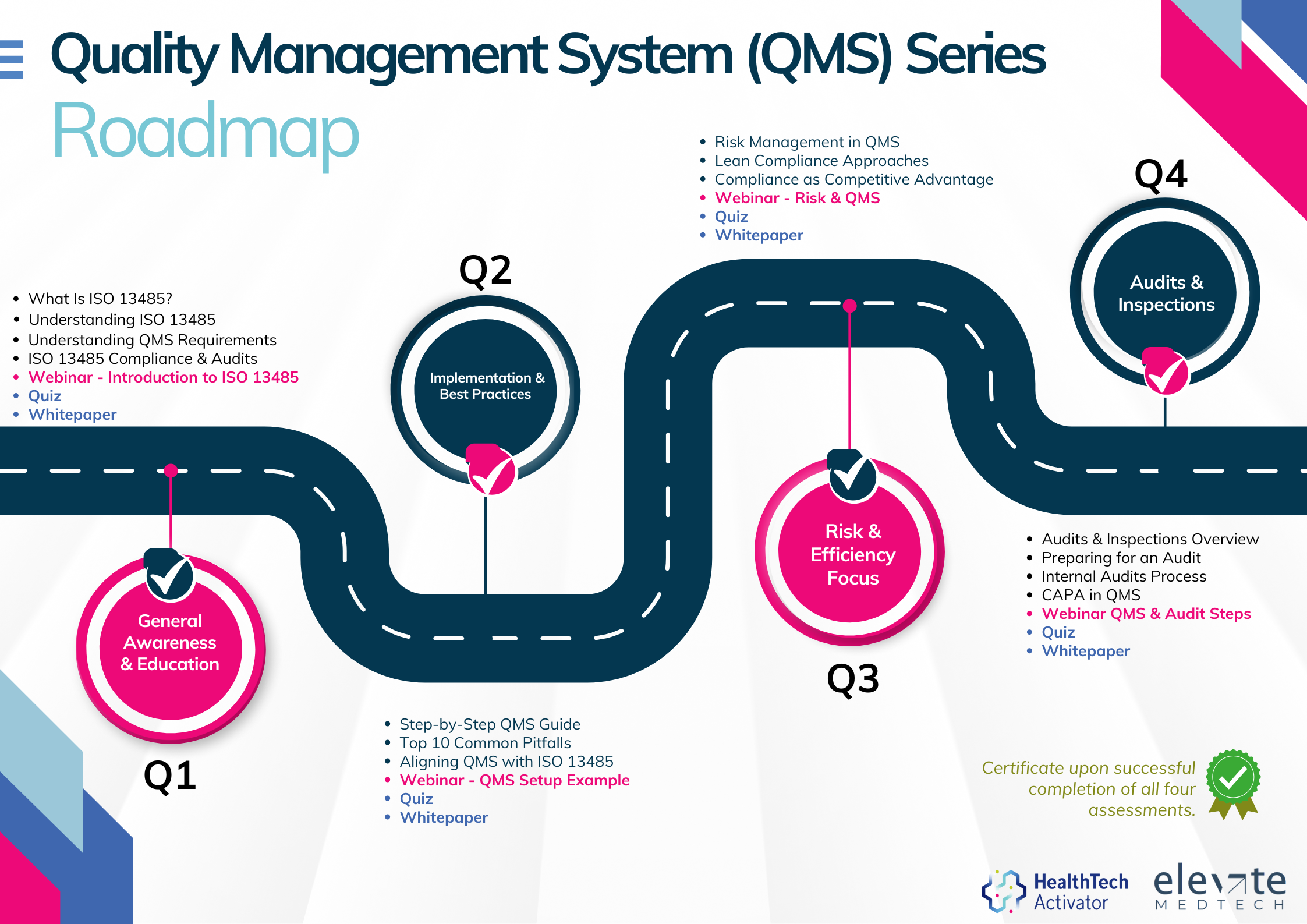
Meeting the requirements of ISO 13485 is one thing, putting them into practice is another. That’s why this article is part of a broader series developed for New Zealand’s HealthTech sector, aimed at helping teams turn regulatory expectations into working systems.
Over 12 months, this series explores ISO 13485 in four parts: from first steps and system setup to risk management and audit readiness. Each quarter combines practical content with interactive workshops to support implementation in real-world settings.